soft water
night_scented_stock
17 years ago
Related Stories

GARDENING GUIDESGreat Garden Combo: 3 Soft-Looking Plants for a Dry Climate
Weave a romantic tapestry with this drought-tolerant combination of plants as tough as they are lovely
Full Story
DECORATING GUIDES'Soft Modern' Style Offers Best of Both Worlds
Mix in a few curves and soft colors but nix the clutter, and the happy result is a balanced new take on modern design
Full Story
KITCHEN DESIGNSoft Hues Create a Calm Mood in a Historic Kitchen
Mellow colors, curved edges and quality materials help this kitchen in a Georgian townhouse feel relaxed while functioning beautifully
Full Story
GARDENING AND LANDSCAPINGHardscaping Shows Its Soft Side
Who says hardscaping has to be hard? Consider these gentle, sustainable and DIY-friendly alternatives
Full Story
FOLIAGEThe Right Touch: 13 Soft, Fuzzy Plants for Gardens and Pots
Brush a hand on velvety foliage or fluffy plumes for a sensory garden experience beyond sight and smell
Full Story
LANDSCAPE DESIGNInside Houzz: Soft Geometry in a Modern Wisconsin Garden
In a city known for harsh winters, homeowners enjoy outdoor living inspired by Southern California
Full Story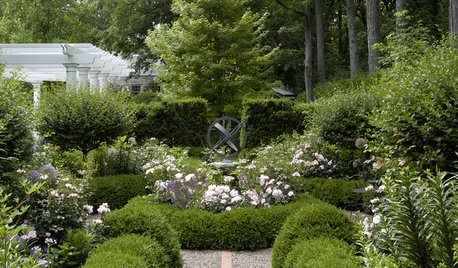
LANDSCAPE DESIGNDefine Your Garden Softly With Planted Borders
Why make things hard for your garden's edges? Embrace a softer side by trading brick and concrete for shrubs, grasses and ground covers
Full Story
BEDROOMSRoom of the Day: Soft, Inviting Decor Suits an Ocean View
A designer focuses on creating an enticing haven for a couple on the coast of Vancouver
Full Story
PRODUCT PICKSGuest Picks: Soft Seating for Kids
These comfy chairs, poufs and beanbags are easy to move and great to look at — and some can even hide a mess of toys inside
Full Story
DECORATING GUIDESRoom of the Day: Softly Elegant Look for a Formal Parlor Room
A design duo adds modern touches that honor historic architectural details while bringing the room up to date
Full StorySponsored
Columbus Area's Luxury Design Build Firm | 17x Best of Houzz Winner!
More Discussions






jenny_in_se_pa
night_scented_stockOriginal Author
Related Professionals
Garden City Landscape Architects & Landscape Designers · Horsham Landscape Architects & Landscape Designers · Surprise Landscape Contractors · Cedar Hill Landscape Contractors · Davis Landscape Contractors · Dedham Landscape Contractors · Homewood Landscape Contractors · Los Banos Landscape Contractors · Mount Kisco Landscape Contractors · Rockwall Landscape Contractors · Vacaville Landscape Contractors · College Station Swimming Pool Builders · Fontana Swimming Pool Builders · Newman Swimming Pool Builders · Tucson Swimming Pool Buildersrhizo_1 (North AL) zone 7
jenny_in_se_pa
rhizo_1 (North AL) zone 7
jean001
luis_pr
jenny_in_se_pa