nutradip Tri-Meter PPM question
chinamon
14 years ago
Related Stories

KITCHEN DESIGNKitchen of the Week: Surprise Storage in Sydney
Hidden appliances and a secret scullery make for a kitchen so streamlined, you might not guess its true purpose
Full Story





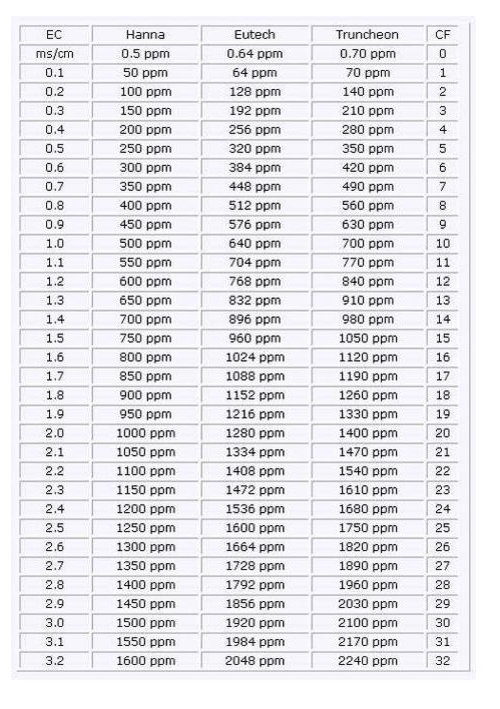

grizzman
chinamonOriginal Author
Related Professionals
Norfolk Landscape Architects & Landscape Designers · Arlington Landscape Architects & Landscape Designers · Citrus Heights Landscape Architects & Landscape Designers · Kapaa Landscape Architects & Landscape Designers · Signal Hill Landscape Architects & Landscape Designers · Milford Landscape Contractors · Concord Landscape Contractors · Brookside Landscape Contractors · Aloha Landscape Contractors · Azalea Park Landscape Contractors · East Chicago Landscape Contractors · Golden Landscape Contractors · Northbridge Landscape Contractors · Rockville Landscape Contractors · Uxbridge Landscape ContractorschinamonOriginal Author
chinamonOriginal Author
lucas_formulas
lucas_formulas
chinamonOriginal Author
chinamonOriginal Author
lucas_formulas
lucas_formulas
urbangardenfarmer
chinamonOriginal Author
lucas_formulas
chinamonOriginal Author
lucas_formulas
urbangardenfarmer
chinamonOriginal Author
urbangardenfarmer
chinamonOriginal Author
lucas_formulas