Testing PPM
hendu2875
10 years ago
Featured Answer
Sort by:Oldest
Comments (17)
biggyboy
10 years agohendu2875
10 years agoRelated Professionals
Richmond Heights Landscape Architects & Landscape Designers · Billerica Landscape Contractors · Stamford Landscape Contractors · Bloomington Landscape Contractors · Eureka Landscape Contractors · La Vista Landscape Contractors · New Baltimore Landscape Contractors · Raleigh Landscape Contractors · St. Louis Landscape Contractors · Vancouver Landscape Contractors · Watertown Landscape Contractors · West Chester Landscape Contractors · Quartz Hill Landscape Contractors · Hueytown Landscape Contractors · Fountain Hills Outdoor Lighting & Audio Visual Systemsgrizzman
10 years agoPupillaCharites
10 years agohendu2875
10 years agoPupillaCharites
10 years agoPupillaCharites
10 years agohendu2875
10 years agohendu2875
10 years agohendu2875
10 years agohex2006
10 years agoPupillaCharites
10 years agohex2006
10 years agohendu2875
10 years agoPupillaCharites
10 years agowillardb3
10 years ago
Related Stories

KITCHEN APPLIANCESWhat to Consider When Adding a Range Hood
Get to know the types, styles and why you may want to skip a hood altogether
Full Story
LANDSCAPE DESIGNNatural Swimming Pools: More Beauty, No Chemicals
Keep your skin and the environment healthy with a pool that cleans itself, naturally
Full Story
GARDENING AND LANDSCAPING12 Naturally Beautiful Hot Tubs
Prefer a no-plastic look for your patio or yard? Wood, stone and concrete make these hot tubs fit right in with nature
Full Story
HOUSEKEEPINGHow to Clean a Glass Shower Door
See which tools and methods will keep those glass shower walls and doors sparkling clean
Full Story








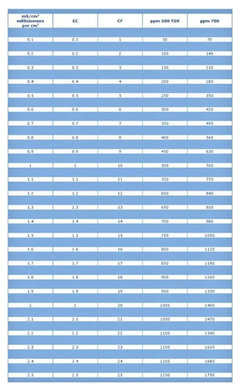

hex2006