Red cabbage pH test of blood meal, corn meal, compost, etc.
I learn my lesson: always test what others recommend, or report. Take Coffee ground, some sites report that as acidic. I tested that with red-cabbage-juice. Initially the color was pinkish (acidic), after 1/2 hour it turned clear, or neutral pH. Coffee ground is a buffer.
I already tested 1 cup of bone meal in the planting hole: disaster ... nearly killed a Gallica band, plus a tomato. And 4 roses with bone meal broke out in B.S., compared to the clean one without.
Blood meal was reported as acidic. I tested it against my alkaline soil (pH 7.7, tested by EarthCo.) .. BLOOD MEAL was almost as blue as my heavy clay, I would put its pH at 7.5.
I tested Encap dry compost sold at Menards for $2 per 18 lb. .. greenish tinge that became clearer with prolonged soaking, most likely a buffer like coffee ground. The last time I tested the bagged compost & manure at HomeDepot, it was more blue than my pH 7.7 clay.
Corn meal was reported in the canning site with pH 7.3. That's for FRESH cooked corn. However, raw, cracked corn stored for years in the bag, underwent anaerobic fermentation & giving off acid, and tested quite acidic. At first it was pinkish, but after 20 minutes, it got darker pink than pine bark. Pine bark pH 4.5, so cracked corn would be around pH 4. Peat moss pH is reported to be 4.
See below from top to bottom, left to right: Blood meal (medium blue), pH around 7.5, compost (just a touch of green, slightly alkaline), corn (very pink) pH around 4, and red-cabbage boiled in distilled water (purple, neutral pH):

This post was edited by Strawberryhill on Fri, Jul 25, 14 at 8:48
Comments (30)
strawchicago z5
Original Author9 years agoHere's a bigger basket of samples: I already tested gritty lime (or pulverized lime), it's bluish-gray, pH above 8. I also tested gypsum (Menards $4.49 for 50 lbs. bag), it turned chalky grayish, so it's neutral pH. Gypsum site reported its pH to be 6.8.
Below picture from L to R, and top to bottom: Potting soil of Rose du Roi, after gritty lime application, plus weeks of constant rain: light blue, or slightly alkaline.
Encap Compost dry granules: tinge of green, became clearer with prolonged soaking. It's a buffer, end result is slightly alkaline.
Bloodmeal: medium blue, pH 7.5
MG regular potting soil: a few sites report it to be 6.5 pH. Mine turned pinkish.
Purple big cup: red-cabbage juice boiled in distilled water, neutral pH.
Cracked corn: very acidic, pH around 4.
Stephen Big Purple in the ground: slightly alkaline
Old Port rose in the ground: composted pine fines mixed with my clay, pH neutral, almost clear-red-cabbage-juice. That's a BS fest, manganese is high in pine fines. Manganese is known as a fungal-promoter in 2 studies: one which manganese promoted fermentation, and the other promoted rust in plants.
Potting soil mixed with red lava: slightly alkaline, around 7.2 with clean Duchess de Rohan rose.
*** Conclusion: I did samples in the past: such as horse manure where it turned VERY DARK blue (pH above 8), or wood ash (bright green, pH above 10), or baking soda (greenish blue, pH near 9).
The EnCap compost is VERY LIGHT green, indicative of its buffering ability, so I don't think it's as harsh-alkaline as the quick lime in horse manure, which turned it DARK BLUE immediately. Horse manure was more alkaline than my clay at pH 7.7 (tested by EarthCo. professional soil testing company).

This post was edited by Strawberryhill on Fri, Jul 25, 14 at 8:55
strawchicago z5
Original Author9 years agoHere's the result of application of gritty lime (pH 9) on own-root Radio Times. The leaves became pale, but the rose shot up with vigor, breaking out in buds. I checked that bush after our month-long rain & high humidity: very clean.
Radio Times was pale like that when it was next to my limestone patio, giving 40+ blooms in spring flush. Then I moved it, and made the soil neutral with pine-barks ... it went down hill, with 2-years of stunt-growth & black spots.
Recently I brought the pH up by spreading gritty lime, and was surprised by its DOUBLING IN HEIGHT & lots of buds & clean foliage ... some roses are healthier at higher pH. I take pale foliage & more blooming over dark-green leaves and BS anytime !!

Related Professionals
Waterbury Landscape Contractors · Downey Landscape Contractors · El Mirage Landscape Contractors · Fair Lawn Landscape Contractors · Flagstaff Landscape Contractors · Gurnee Landscape Contractors · Hurricane Landscape Contractors · Inglewood Landscape Contractors · Point Pleasant Landscape Contractors · Post Falls Landscape Contractors · Rockwall Landscape Contractors · San Pedro Landscape Contractors · Weslaco Landscape Contractors · Woodburn Landscape Contractors · San Pablo Landscape Contractorsstrawchicago z5
Original Author9 years agoHere's and old-garden-rose which I dug up to fix the soil. It didn't bloom for 1 year, so I dug it up, and found rock-hard clay, pH near 8. I fixed it with cracked corn (pH 4), gypsum (neutral pH), red lava rock for potassium & iron. I NO longer use pine bark to fix my clay, after witnessing roses with pine-bark in the hole have more B.S.. Pine bark is high in manganese, a fungal-promoter.
Here's the result: very perky and darker green leaves: Le Nia Rias, a Centifola rose:

jim1961 / Central Pennsylvania / Zone 6
9 years agoGreat info Strawbhill!
I spread alittle used coffee grounds once in awhile just to increase earthworm population.
My way of thinking is if I increase earthworm populations a bit they will keep the soil tilled and their castings should benefit too...Do you have any info on earthworms Strawbhill?
strawchicago z5
Original Author9 years agoHi Jim: In my last house of acidic clay (blue hydrangeas), I didn't see any earthworms. But in my current house of alkaline dolomitic clay, tons of them.
I think the magnesium content is what attract earthworms, rather than soil pH. When I tested cocoa mulch at pH 5.8, high in nitrogen, potassium, calcium & magnesium ... there was TONS of earthworm, like 10 per scoop. My current soil is tested exceedingly HIGH in magnesium.
In our recent months of rainy weather, I spreaded gritty dolomitic lime on some roses ... they hated it and turned pale. So I scraped it off, and found lots of earthworm underneath. Coffee is high in magnesium (helps folks with constipation!) ... thus coffee grounds would be beneficial for earthworms.
Not sure why cracked corn, at such low pH of 4 works against fungal diseases, until I checked the soil-research, see below excerpt: "ABSTRACT
The influence of pH on the two principal decomposer groups in soil, fungi and bacteria, was investigated along a continuous soil pH gradient at Rothamsted Research in the United Kingdom. This experimental location provides a uniform pH gradient, ranging from pH 8.3 to 4.0, within 180 m in a silty loam soil. .. The growth-based measurements revealed a fivefold decrease in bacterial growth and a fivefold increase in fungal growth with lower pH. .. Below pH 4.5 there was universal inhibition of all microbial variables."So Corn meal, being acidic at pH 4, would suppress BOTH bacteria and fungi. In contrast, dolomitic lime at pH 9 is known to encourage growth of beneficial bacteria and earthworms. For fungal suppression, nothing beats woodash ... folks reported using it successfully against mildew and rust. Some info:
pH woodash is 10.4 versus pH of limestone is 9.9
Woodash is high in calcium, potassium, zinc, chromium, and all trace elements . Woodash also contains 123 mg/kg of Boron, which is vital for plant growth. Boron is less available in alkaline clay.
Woodash has 70 mg/kg of copper, a fungicide in Bordeux mixture. It has 233 mg/kg of zinc (compared to 113 in limestone) a fungicide fraction in Mancozeb spray. It has boron, another fungicide for dry rot. It has 65 mg of lead, compared to 55 mg of lead in limestone, also a fungicide. Finally woodash has 57 mg of chromium, another fungicide, here's a quote:
"In the past, chromium was also used in cooling towers as a rust and corrosion inhibitor and as a fungicide. "
http://corrosion-doctors.org/Elements-Toxic/Chromium.htmBrewer's yeast is high in chromium and selenium, but with acidic pH at 5. Austin own-root Eglantyne prefers it acidic, so it blooms well with Brewer's Yeast & healthy in this month-long rain:

This post was edited by Strawberryhill on Sat, Jul 19, 14 at 9:42
jim1961 / Central Pennsylvania / Zone 6
9 years agoThanks for the info... Yes the rose I put Brewers Yeast under is doing well also... :-)
Now I'm not sure if you read my other post on another thread or not but I dug out both Carefree Sunshines bagged them and discarded.
I decided to discard because no other roses were showing signs of rose midge. So hopefully this slows it down or ends it!
I have my suspicions that the Rose Midge were in the potted soil of the roses when they came from the vendor.
So I can not report on what Brewers Yeast did for them since they are gone now.strawchicago z5
Original Author9 years agoHi Jim: Thanks for the info., I did not realize that you dug BOTH of them ... I thought only one. I'm sorry for your losses, but better safe than sorry.
Rock-hard clay soil doesn't hold the optimal moisture level, but fluffy soil does. I DID NOT have rose-midge in my last garden of acidic soil, nor my current alkaline clay until I fixed Golden Celebration rose with fluffy potting soil & pine barks .. then I got rose midge on that one. Plus it's in a very shady area. I repost the info. on rose midge:
Experiments at Cornell University stated, " Laboratory results indicated that extremely dry and extremely wet soil hinders swede midge emergence. Optimal moisture content for swede midge emergence was from 25 ��" 75 %, and varied in different soils."
That explains why I don't have rose midge in my rock-hard clay. My heavy clay is sticky-wet when it's rained, and rock-hard when dry. 15 minutes from me is Cantigny rose park, with 1,200 roses. They use zero mulch, just bare dirt. But when people mulch with bark, or horse manure on a fluffy bedding ... that retains optimal moisture level longer for midge germination.
More from Cornell University: "These results suggest that cultural practices, such as flooding fields during non-cropping periods to achieve 100% soil moisture level or even drying the soil, may be viable methods to reduce swede midge emergence. Similarily, swede midge populations and damage are expected to be REDUCED when saturated soil or drought conditions occur."
eHow recommended that for rose midge, removing the top soil, and putting new soil in late season will stop midge from germinating next year. That's what I did in zone 5a for winter-protection: I dump new soil in late fall, to protect my roses. The bagged soils here are alkaline clay, pH near 8.
jim1961 / Central Pennsylvania / Zone 6
9 years agoI will just leave our soil next year uncovered and see how things go... No mulch or compost will be applied. Once the roses bushes fill out with leaves it keeps the roots shaded anyhow.
strawchicago z5
Original Author9 years agoHi Jim: Best wish to your no-mulching roses for next year. My clay germinate seeds too well, so I won't use cracked corn in the planting hole. Doing that late fall was OK, since my cold zone 5a winter killed the corn's germination in the planting hole. WARNING: cracked corn germinate into plants .. not recommended.
I once read that magnesium helps with seed-germination. So I tested that: This week I put 1/2 cup cracked corn, plus 3/4 cup of my sticky clay (high in magnesium) in potting soil. COMPARE THAT to 1/2 cup cracked corn in MG potting soil alone.
After 1 week of constant rain, there were at least 20 baby-corn-plants in the pot with clay mixed in. But less than 5 corn-germination in the pot with Moisture-control potting soil alone.
I tested the growth rate of roses in potting soil, with, and without some clay mixed-in. Impressive moisture-retention with the clay mixed in, that's when Sonia Rykiel gave me 15+ blooms in dinky plastic pot, at temp. above 90 degrees.
That's the logic for the Pro-Mix potting soil I saw at Menards, sold for $6 per pack. It has peat moss, perlite, dolomitic lime, and gypsum. Dolomitic lime is 25% calcium and 10% magnesium. My clay is tested exceedingly high in magnesium, and barely adequate in calcium.
I have 5 bands coming from Heirloom, will test their growth rate in potting soil with a tiny bit of native clay mixed in for magnesium, besides the 1/2 cup of gypsum that I added.
Result of no-mulch in my garden? Flowers germinate themselves from seeds of last year. The snapdragon and yellow calendula sow-seed themselves, the alyssum I have to save the seeds indoor through the winter.

This post was edited by Strawberryhill on Mon, Jul 14, 14 at 22:50
Slimy_Okra
9 years agoActually, it isn't that simple. The protein in blood meal converts to ammonia, which is alkaline. However soil nitrifying bacteria convert this to nitric acid. So blood meal and other organic nitrogen fertilizers are ultimately acidic.
Don't put too much importance on the initial pH of a product. Soil chemistry is far more complex than a litmus test.
strawchicago z5
Original Author9 years agoThank you, Slimy_Okra. That's a great user name, I like that !! I check the info. you gave, and it's correct. You are right that the initial pH of a product will change, given time, and other factors: water, light, and soil bacteria.
I took a soil sample from the rose park nearby. At first it was pinkish in red-cabbage juice, after 1/2 hour, it was blue, a bit lighter than my soil at pH 7.7 (tested by EarthCo.). There are a few pale roses at the park, similar to my garden. TIME is a big factor for elements in the soil to stabilize in water.
The below link explains how various factors change the form of ammonia " Ammonia-nitrogen (NH3-N) has a more toxic form at high pH and a less toxic form at low pH, un-ionized ammonia (NH3) and ionized ammonia (NH4+), respectively. In addition, ammonia toxicity increases as temperature rises."
Here is a link that might be useful: Daily pH cycle and Ammonia toxicity
strawchicago z5
Original Author9 years agoI did more red-cabbage testing today. See picture below:
From top to bottom on leftmost side: river-birch bark, reddish pink, very acidic, pH below 4. Rain water (pinkish purple, pH around 6.5). East coast rain water is known to be more acidic than my Chicagoland. Distilled water (purple, neutral pH). Last is my hard-well tap-water, pH around 8.3. I double-checked this value with fish-tank litmus paper sold at Walmart.
Next column, from top to bottom: Composted willow-branches, neutral pH, whole-wheat flour (acidic pH 5), red-lava rock (slightly alkaline, pH 7.2), my fixed clay with leaves (pH 7.4), last is pine bark, almost red & very acidic pH 4.
Last column, from top to bottom: Milorganite, pH 7.7, blue like my native clay (tested by EarthCo. professional soil company). Organic cracked corn, acidic, pH 5. Brewer's yeast, even more acidic, pH below 5. Bluish-green container at raised height is baking soda, pH 9. Last sample is composted manure from Menards, slightly alkaline 7.4 ... same color as my clay fixed with leaves.

strawchicago z5
Original Author9 years agoHere's a close up to show how alkaline my tap water at pH 8.5, compared to baking soda at pH 9. Leftmost column, from top to bottom: Rain water, slightly acidic, pH 6.8 (east coast rain water is more acidic at pH 5.6). Distilled water, neutral pH. Last is my hard-well tap water, pH 8.3 to 8.5. Compare that to the raised sample of baking soda, at pH 9 next to it.
Next column, from top to bottom: Genetically modified crack corn, almost red at pH 3, MORE ACIDIC than the organic corn. Next is Red lava rock & my clay tested slightly alkaline, pH 7.2. Baking soda bluish-green, pH 9. Last sample is pine bark, pH 4.5.

This post was edited by Strawberryhill on Fri, Jul 18, 14 at 21:50
strawchicago z5
Original Author9 years agoBelow picture shows how acidic Brewer's yeast is. From top to bottom: organic cracked corn (pH 4), brewer's yeast (pH 5), Milorganite, pH 7.7, gets darker blue as it soaked for 20 minutes. Last is composted manure from Menards, slight blue & almost clear which shows some buffering ability.
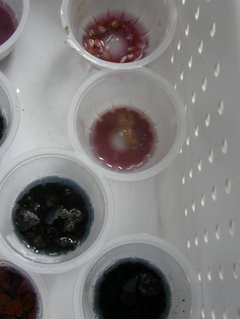
strawchicago z5
Original Author9 years agoHere's the acid-group. I don't have vinegar sample there, but it's fuchsia pink (gaudy-reddish pink). From Answers.com: "commercial distilled white vinegars contain 5-10% acetic acid and have a pH roughly around 2.40 - 3.40."
Left column, from top to bottom: Composted willow branches is same color as rain water, pH 6.5. Paper-birch is acidic, pH 4.5. Pine bark is VERY ACIDIC, pH 4. Left of pine bark is rain water (pH 6.5 to 6.8).
Next column from top to bottom: whole-wheat flour is acidic, pH 5. Organic popcorn pH 4, and Brewer's yeast pH 5.

This post was edited by Strawberryhill on Fri, Jul 18, 14 at 21:52
strawchicago z5
Original Author9 years agoBelow shows how a tiny bit of used-lemon-rind can acidify my very alkaline tap (pH 8.3). To bring my tap-water to the same color as rain water, it's 1/4 used-lemon-rind soaked in 5-gallons bucket of water. Advantage of lemon: it provides vitamin C. There are several studies that showed vitamin C is essential for plant growth.
Leftmost column, top to bottom: My tap water, pH 8.3. Middle is tap water with a tiny-bit of used-lemon, pH 4. Last is neutral pH (red-cabbage boiled in distilled water).
Rightmost column, top to bottom: Milorganite, pH 7.7, dark blue. Tomato-Tone, clear juice above, neutral pH, a buffer. Whole wheat flour, pH 5.

strawchicago z5
Original Author9 years agoI did more testing with red-cabbage juice today with: gritty lime (bright blue, pH 9). Gypsum (chalky & cloudy, its reported pH is 6.8 by the gypsum site), Comte de Chambord hole, which was previously occupied by Annie L. McDowell, which I lowered the soil pH with sulfur ... Comte has BAD blackspots, so I put tons of alkaline Encap Compost granules, and the soil is still slightly acidic, or pinkish in red-cabbage juice.
Previous hole occupied by Firefighter rose, which died this past coldest winter: also slightly acidic. I also test a big chunk of my rock-hard alkaline clay, plus a piece of pine bark together ... it came out slightly blue, around pH 7.2. I also tested bone meal, came out to be neutral, or clear in red-cabbage juice. I tested Maca powder. Maca is a health food powder high in iodine, B-vitamins, and copper ... it's acidic like Brewer's yeast.
Will have to put gritty lime in Comte's hole to fix its severe black spots.
strawchicago z5
Original Author8 years agoIt's best to do soil-test at root-level, at least 8 inches below ground if you are in a cold zone and plant your roses DEEP like me. Plus roots DO SECRET ACID which lower the soil pH. Radio Times was next to a tree, and that area is fluffy & loose, thanks to secretion of acid from the tree's root nearby. So when I topped Radio times with gritty lime, it worked wonder in RAISING the pH, stopped the black spots & promote clean growth.
The opposite happened with Austin roses in rock-hard alkaline clay, at pH 7.7. Austin roses as own-root DO NOT SECRET MUCH acid like Dr. Huey, So own-root Jude the Obscure was really wimpy in my rock-hard clay, since it cannot secret enough acid to go through rock-hard-clay. Dr. Huey-root-stock is different, he can go through my rock-hard clay better than my shovel. When I dug up a knock-out GRAFTED ON DR. HUEY, the root extended 4 feet away to steel water from my annual flowers.
For soil test, it's best to get the soil DEEP DOWN where the root of a particular rose, you will find your soil pH varies with different parts of the garden. When I tested 10 different places in my garden, they all varied: Rock-hard clay at high pH where no plant grows. Rock-hard clay at high pH where rain water can't reach (under the roof's edge). But soft & fluffy soil at neutral pH where lots of rain water (under the rain-spout). And really soft soil when I dug up Dr. Huey ... same with the soil where I dumped vinegar to kill weeds.
msdorkgirl
8 years agolast modified: 8 years agoHmm, I want to put Orchid Bark on top of all my potted roses like the Remember Me Rose because it seems to be doing well ... would you just put wood bark in a cup overnight and then test the resulting liquid for PH?

Remember Me (12/2014 -- I overused Marine Cuisine and panicked when the leaves were so shiny and large, but note the wood chips --- it remains still very bushy but now normal sized leaves so must be wood bark helping it)

Lasting Love repotted with nutritious mix to see if it will maybe even give me some sucker of Huey, I'd be maybe happy with that
strawchicago z5
Original Author8 years agolast modified: 8 years agoWow! I love the shiny leaves of your Remember Rose. Orchids like roses like it slightly acidic, with the ideal water at pH 6.5 http://www.herbs2000.com/flowers/o_watering.htm
I found the pH of orchid bark in the below link, with a picture that looks just like your bark in pots. pH 5.0-5.5 http://playbark.com/content/orchid-bark
For pots, when alkaline tap water passes through the orchid-bark, the pH of tap water will be neutralized. Pots drain well, so that works. However, in my last house of acidic clay, poor drainage, THICK pine-bark (at pH 4) didn't work well with heavy rain. Folks in Fig forum tested pine-bark soaked in rain water, and the pH dropped to below 3. Pine bark is more acidic & darker color than orchid bark, pine bark doesn't decompose well ... still have chunks of pine bark in my garden that are 8 years old.
Orchid bark at pH above 5 is safer than pine bark. I mulched pots with cocoa mulch before, at pH 5.4, and it worked great with shiny leaves.
strawchicago z5
Original Author8 years agolast modified: 8 years agoOrganics make leaves shiny, like Marine Cuisine with NPK 10-7-7 (crab meal, shrimp meal, seabird guano, and kelp, plus chemical nitrogen). I get shiny leaves with cracked corn and alfalfa hay in the planting hole, as in Radio Times, zero black spots, picture taken today July 15, after months of constant rain. I also topped with red-lava-rock to buffer against the acidity of rain (pH of rain is 5.6). Lots of buds on Radio Times rose for 2nd flush:

- strawchicago z5 thanked msdorkgirl
sharon2079
3 years agolast modified: 3 years agoI am trying to the ph of my soil.
I bought the red cabbage.

and distilled water
I was surprised that the water in my tap was pretty much the same color.as the distilled water.... though I thought the distilled water was suppose to be a different color.... more red.


Here is a picture of the dirt before I started. Notice one is much sandier than the other one. They are all amended the same but somehow the amended soil turns sand by the end of the summer....
A
I was actually surprised it was sand already. I had not noticed it was so sandy, but found it that way after I pulled back the mulch.
I do mulch with banyan leaves. This time of year though I mulch with the flowers from a sausage tree. The flowers are dark maroon. It was the only one that had a pink color. The rest are dark.... like black one is gray.... but I am not sure if the red is because it is acidic or because the flower is also used as dye.




some how the picture of the sand vs dirt showed up out of order.
strawchicago z5 thanked sharon2079strawchicago z5
Original Author3 years agolast modified: 3 years agoSharon: THANK YOU FOR THOSE FANTASTIC PICTURES !!
I also did red-cabbage test today Sat. 7/25/20. Distilled water boiled in red cabbage is actually acidic according to on-line info. "Pure distilled water should be neutral with a pH of 7, but because it absorbs carbon dioxide from the atmosphere, it's actually slightly acidic with a pH of 5.8." From your pictures above, the grayish blue solution is alkaline with pH 7.5. My pH 7.7 clay is a bit bluer (tested by Earth Corp. soil testing company).
Black, gray, or clear solution is neutral pH .. means it's a fantastic buffer (good for plants). I tested COMPOSTED grass clippings and it's clear water above black solution. Buffer is great to neutralize the acidity of rain. Clay buffers better than sand/loamy soil, but the best buffer is composted organic matter. Coffee is also a buffer (at first pinkish, but after 20 min., it's a clear solution).
COMPOSTED plant-matter is very alkaline and neutralizes acidic rain well. COMPOSTED leaves decompose to alkaline pH, when I stuffed a bunch of leaves in LOAMY & fluffy soil .. the next year that got converted into HARD CLAY, very alkaline. Green grass clippings stay fluffy longer (more nitrogen & more acidic). Brown leaves are considered "carbon" in a compost pile, and decompose to alkaline pH & hard like clay.
My tap-water in red-cabbage is VERY BLUE, it's at pH 9 as stated on village's website. I also tested baking soda and it's more blue than my tap-water.
I tested paver's sand or yellow coarse sand, and it's slightly alkaline.
I tested composted manure and it's very alkaline (from the lime added to deodorize and kill weeds in the bag). The pH of composted manure is just as alkaline as my clay at pH 7.7.
Your LAST pic. is slightly acidic. Rain-water is even more acidic & reddish purple. Rain water here is pH 4.5. I also tested some drops of vinegar and it's fuchsia red around pH 3.
All my rooting-soil for cuttings are black or clear solution at neutral pH (they have lots of yellow sand or vermiculite mixed in).
The clay taken from diseased & black spotted roses are slightly acidic, rather than blue like my pH 7.7 clay. I had tested 5 different roses with black spots in the past, and the soil taken from the root level is pinkish in red-cabbage juice. It's either acidic rain can't drain well from that spot, or else there's NOT enough buffer to neutralize the heavy rain going down.





















strawchicago z5Original Author